| 品牌 |
货号 |
纯度标准 |
包装 |
价格/优惠价 |
折扣价 |
特价 |
上海 | 济南 | 成都 | 武汉 | 天津 |
数量1 |
购买 |
| 韶远 |
SY004517 |
>98% |
1g |
40.00/0 |
0 |
- |
现货 |
- |
- |
现货 |
- |
|

|
| 韶远 |
SY004517 |
>98% |
5g |
51.00/0 |
0 |
- |
- |
- |
- |
- |
- |
|

|
| 韶远 |
SY004517 |
>98% |
10g |
92.00/0 |
0 |
- |
- |
- |
- |
- |
- |
|

|
| 韶远 |
SY004517 |
>98% |
25g |
222.00/0 |
0 |
- |
- |
- |
- |
- |
- |
|

|
| 韶远 |
SY004517 |
>98% |
100g |
748.00/0 |
0 |
- |
- |
- |
- |
- |
- |
|

|
| 韶远 |
SY004517 |
>98% |
1000g |
POA/0 |
POA |
POA |
- |
- |
- |
- |
- |
|

|
|
|
产品信息安全信息参考文献
|
闪点
|
|
|
沸点
|
|
|
密度
|
|
|
储存条件
|
|
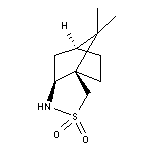
Versatile chiral auxiliary introduced by Oppolzer. Review: Tetrahedron, 49, 293 (1993). N-Acryloyl derivatives undergo enantioselective cycloaddition reactions: Helv. Chim. Acta, 67, 1397 (1984); Tetrahedron, 43, 1969 (1987). Hydrogenation of N-acryloyl derivatives proceeds with high diastereoselectivity: Tetrahedron Lett., 27, 183 (1986); Helv. Chim. Acta, 69, 1542 (1986), 69, 1817 (1986); 70, 1666 (1987). Induces asymmetric conjugate addition of Grignard reagents to N-acryloyl derivatives: Helv. Chim. Acta, 70, 2201 (1987). In the presence of strong bases, N-acyl derivatives undergo stereoselective alkylation: Tetrahedron Lett., 30, 5603 (1989); or aldol-type condensation reactions with aldehydes: J. Am. Chem. Soc., 112, 2767 (1990); Tetrahedron Lett., 32, 61 (1991). Chiral auxiliary for the asymmetric Baylis-Hillman reaction: J. Am. Chem. Soc., 119, 4317 (1997).